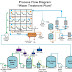
Kata Kunci : Minyak Bumi, Refinery, Fossil Fuel, Fraksinasi, Cracking, Crude Oil, Bilangan Oktan
Energi yg paling banyak kita gunakan utk transportasi adalah bensin. Dan tahukah anda bahwa bensin ini memiliki spesifikasi yg bermacam-macam, contohnya premium, pertamax, pertamax plus. Perbedaan dari ketiganya adalah Bilangan Oktan yang artinya kemampuan bahan bakar utk menahan knocking (ketukan) dan terbakar scr spontan, juga bisa diartikan nilai perbandingan antara isooktana dengan n-heptana. Isooktana adalah hidrokarbon dalam minyak bumi yg sangat bagus karena tidak mudah terbakar scr spontan dan beda dengan n-heptana yg sangat mudah terbakar dg sendirinya. Isooktana mempunyai struktur rantai bercabang dan n-heptana rantai lurus. Semakin tinggi isooktana maka semakin bagus kualitas bensin. Sering juga utk menaikkan oktan, mengurangi knocking dan menghindari polusi ditambahkan TEL (Tetra Ethyl Lead), MTBE (Methyl Tertiary Butyl Ether).
"Most of the energy we use for transportation is gasoline. And did you know that gasoline has kind of specification, examples premium, pertamax and pertamax plus. Differences of that is Octane Number mean ability fuel to hold knocking and flammable spontaneous, also mean rasio number between isooctane with n-heptane. Isooctane is the best petroleum hydrocarbon due to not easy flammable and this very different with n-heptane which very easy flammable by self. Isooctane have branch chain structure and n-heptane straight structure. Heigher of isooctane better gasoline quality. Often to raise octane, reduce knocking and avoid pollusion, added TEL and MTBE."
Energi yg paling banyak kita gunakan utk transportasi adalah bensin. Dan tahukah anda bahwa bensin ini memiliki spesifikasi yg bermacam-macam, contohnya premium, pertamax, pertamax plus. Perbedaan dari ketiganya adalah Bilangan Oktan yang artinya kemampuan bahan bakar utk menahan knocking (ketukan) dan terbakar scr spontan, juga bisa diartikan nilai perbandingan antara isooktana dengan n-heptana. Isooktana adalah hidrokarbon dalam minyak bumi yg sangat bagus karena tidak mudah terbakar scr spontan dan beda dengan n-heptana yg sangat mudah terbakar dg sendirinya. Isooktana mempunyai struktur rantai bercabang dan n-heptana rantai lurus. Semakin tinggi isooktana maka semakin bagus kualitas bensin. Sering juga utk menaikkan oktan, mengurangi knocking dan menghindari polusi ditambahkan TEL (Tetra Ethyl Lead), MTBE (Methyl Tertiary Butyl Ether).
"Most of the energy we use for transportation is gasoline. And did you know that gasoline has kind of specification, examples premium, pertamax and pertamax plus. Differences of that is Octane Number mean ability fuel to hold knocking and flammable spontaneous, also mean rasio number between isooctane with n-heptane. Isooctane is the best petroleum hydrocarbon due to not easy flammable and this very different with n-heptane which very easy flammable by self. Isooctane have branch chain structure and n-heptane straight structure. Heigher of isooctane better gasoline quality. Often to raise octane, reduce knocking and avoid pollusion, added TEL and MTBE."
Pertamax '92 artinya dalam bahan bakar tsb tersusun dari 92% isooktana dan 8% n-heptana. Adapun spesifikasi dari bensin adalah
- Premium bilangan oktannya '80 - 82
- Pertamax '91-92
- Pertamax Plus '95-98
Bensin dengan oktan tinggi akan terbakar lambat apabila terkena tekanan shg irit BBM. Pemilihan bahan bakar agar efisien dan ramah lingkungan terus diupayakan shg kandungan terbanyak nantinya adalah isooktana dan menekan n-heptana, caranya akan dijelaskan dibawah ini.
Di bidang teknik kimia kita seharusnya harus paham tentang pengolahan minyak bumi, bagaimana bahan bakar diperoleh. Masih ingat dalam SMA dulu saya menghafalkan urutan fraksi minyak bumi dari yang teringan ke terberat ( L Be Na Ke So Min ) yaitu LPG, BEnsin, NAfta, KErosin, SOlar, MINyak pelumas. Prosesnya sebagai berikut :
"Gasoline with height octane will flammable slowly if exposed pressure so that more economical fuel. The selection of fuel in order to efficient and environmental friendly continue done so the biggest content is isooctane and reduce n-heptane. The methods will I explain below : In the field of chemical engineering we must understand about petroleum processing, how fuel is got. The processing like below :"
"Gasoline with height octane will flammable slowly if exposed pressure so that more economical fuel. The selection of fuel in order to efficient and environmental friendly continue done so the biggest content is isooctane and reduce n-heptane. The methods will I explain below : In the field of chemical engineering we must understand about petroleum processing, how fuel is got. The processing like below :"
- Distilasi Bertingkat / Fraksinasi
Minyak bumi yg didapat dari pengeboran lepas pantai masih berupa cairan kental (crude oil) yang mengandung komponen-komponen lain seperti logam, belerang, nitrogen, air, dan garam-garam. Maka perlu dilakukan pembersihan yg disebut Desalting (menghilangkan garam-garam yg terikut dg mencampurkan minyak + air, shg garam akan terlarut, selain itu juga mencucinya dengan asam basa shg komponen selain hidrokarbon akan hilang). Setelah itu dilakukan pemasakan dgn furnace pada suhu 370 - 400
°C
, uap yang dihasilkan dimasukkan lewat kolom feed (tempat masukan) menuju menara distilasi. Menara distilasi memisahkan komponen berdasarkan titik didihnya. Menara distilasi didesain seperti yg sudah saya jelaskan di postingan lalu "desain kolom pemisah distilasi". Uap yang masuk akan bergerak keatas, dan semakin keatas suhu menara semakin rendah, shg komponen yg mempunyai titik didih rendah akan terus bergerak keatas dan masuk pada kolom distilat teratas dan komponen dg titik didih tinggi akan mengisi kolom distilat bawah. Fase yg bukan uap akan tertampung pada kolom bagian bawah sebagai residu. Selanjutnya setelah melewati kolom distilat dilakukan kondensasi menggunakan refrigerant.
"1. Fractional Distillation
Petroleum is got from offshore drilling still viscous fluid (crude oil), which contain components such as metals, sulfur, nitrogen, water and salts. It is necessary done cleaning "Desalting" (remove salts entrained with mixing oil + water, so salt will dissolved, and also washing with acid - base so component except hydrocarbon will be lost). After it is done cooked with furnace at temperature 370 - 400 °C, vapor is released will entered through feed column to distillation tower. Distillation tower separated component based on boiling point. Distillation tower is designed like my explain at my post ago about "separation distillation column design". Vapor is entered will move up and higher up column temperature will low so component with low boiling point will continue move up and entry at upper distillate column and component with high boiling point will contain low distillate column. Phase not vapor will collected at low column as residue. An then after through distillate column done condensation using refrigerant."
"1. Fractional Distillation
Petroleum is got from offshore drilling still viscous fluid (crude oil), which contain components such as metals, sulfur, nitrogen, water and salts. It is necessary done cleaning "Desalting" (remove salts entrained with mixing oil + water, so salt will dissolved, and also washing with acid - base so component except hydrocarbon will be lost). After it is done cooked with furnace at temperature 370 - 400 °C, vapor is released will entered through feed column to distillation tower. Distillation tower separated component based on boiling point. Distillation tower is designed like my explain at my post ago about "separation distillation column design". Vapor is entered will move up and higher up column temperature will low so component with low boiling point will continue move up and entry at upper distillate column and component with high boiling point will contain low distillate column. Phase not vapor will collected at low column as residue. An then after through distillate column done condensation using refrigerant."
Fraksi hidrokarbon yg didapatkan mulai dari kolom distilat teratas adalah :
 |
| Distilasi Fraksinasi |
Pada tahap ini fraksi minyak bumi bisa direkayasa untk menghasilkan jenis bahan bakar. Misalnya saja ingin bensin yg banyak dg jumlah karbon antara 5 -10.
"At this stage fraction of petroleum can be engineered to produced kind of fuel. Such as want much gasoline with carbon number 5 - 10"
Ada 3 cara cracking :
- Thermal Cracking
"The process is called pyrolisis (using fire) is hydrocarbon alkane passed at column with high temperatures ( 700 - 900 °C ) and quickly cooled so happen shortening alkane chain (convert to alkene + hydrogen). Also can be interpreted petroleum fraction with large atomic number C want to crack in smal atomic number."
- Catalytic Cracking
Proses penggunaan katalis dan biasanya SiO2 dan Al2O3
- Hydro Cracking
"The combination between both and produce saturated compound, performed by high pressure"
3. Reforming
Pengubahan struktur molekul dari yg semula lurus menjadi bercabang, utk memperbaiki kualitas dari bahan bakar (bensin) karena isooktana (bercabang) lebih baik daripada n-heptana (lurus). Prosesnya dengan menggunakan katalis dan pemanasan suhu tinggi.
"Convert molecular structure from straight to branch chain, to improve quality of fuel (gasoline) due to isooctane (branch) better than n-heptane (straight). Process using catalyst and using high temperature heating."
4. Polimerisasi
Penggabungan molekul-molekul sederhana menjadi molekul komplek. Misalnya isooktana hasil penggabungan antara isobutana dan isobutene.
"Fusion of simple molecules to complex molecules. Such as isooctane result of fusion between isobutane and isobutene."
5. Treating
Pemurnian minyak bumi dg penghilangan pengotor-pengotornya, misalnya penambahan soda kaustik (NaOH).
"Refining of petroleum with removal of impurities, such as addition caustic soda."
6. Blending
Proses penambahan zat aditif, misal pada bensin ditambah TEL.
"The process adding additives, such as gasoline added TEL"
Dari proses diatas akan didapatkan bahan bakar fosil yang telah bisa kita dapatkan di SPBU.
Referensi : Diolah dari berbagai sumber
ARTIKEL TERKAIT :
1. Desain Kolom Pemisah Distilasi
2. Macam - Macam Reaktor (Reactor)
3. Macam - Macam Proses Pemisahan (Separation Process)
4. Hukum Kimia - Fisika & Diagram Untuk Air pada Berbagai Kondisi
5. Pemilihan Desain Efektif Reaktor
ARTIKEL TERKAIT :
1. Desain Kolom Pemisah Distilasi
2. Macam - Macam Reaktor (Reactor)
3. Macam - Macam Proses Pemisahan (Separation Process)
4. Hukum Kimia - Fisika & Diagram Untuk Air pada Berbagai Kondisi
5. Pemilihan Desain Efektif Reaktor
Previous
« Prev Post
« Prev Post
Next
Next Post »
Next Post »




.jpg)